An element named after a continent is a chemical element whose name is derived from the name of a continent. For example, the element americium was named after the Americas.
There are several reasons why elements might be named after continents. In some cases, the element was first discovered on that continent. For example, europium was first discovered in Europe. In other cases, the element was named after a country or region on that continent. For example, francium was named after France.
The names of elements can provide us with valuable insights into the history of chemistry and the world around us. By understanding the origins of element names, we can gain a better appreciation for the scientific process and the people who have contributed to it.
Element named after a continent
Elements named after continents are a testament to the global nature of scientific discovery. They represent the contributions of scientists from all over the world to our understanding of the chemical elements.
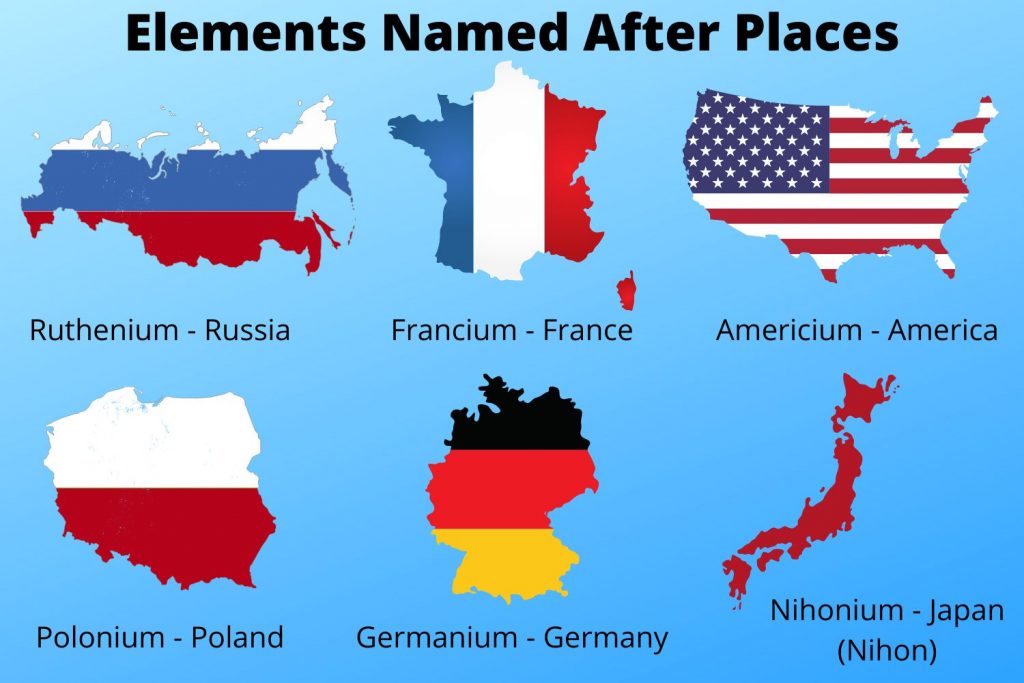
- Americium - Americas
- Europium - Europe
- Francium - France
- Germanium - Germany
- Polonium - Poland
- Rutherfordium - Rutherford, New Zealand
- Dubnium - Dubna, Russia
- Seaborgium - Glenn T. Seaborg, USA
These elements have a wide range of applications, from medical imaging to nuclear power. They are a reminder that science is a global enterprise, and that the pursuit of knowledge benefits all of humanity.
Americium - Americas
Americium is a radioactive chemical element with the symbol Am and atomic number 95. It is named after the Americas, where it was first discovered in 1944. Americium is a member of the actinide series, and is the heaviest element that can be found naturally in significant amounts. It is a silvery-white metal that is malleable and ductile.
Americium is produced by the neutron bombardment of uranium in nuclear reactors. It is used in a variety of applications, including smoke detectors, neutron sources, and medical imaging. Americium-241 is the most common isotope of americium, and it is used in smoke detectors. Americium-243 is used in neutron sources, and americium-242m is used in medical imaging.
Americium is a valuable element with a wide range of applications. It is a testament to the power of scientific discovery, and it is an important part of our modern world.
Europium - Europe
Europium is a chemical element with the symbol Eu and atomic number 63. It is a member of the lanthanide series, and is named after Europe, where it was first discovered in 1896. Europium is a silvery-white metal that is malleable and ductile.
- Discovery
Europium was discovered by the French chemist Eugne-Anatole Demaray in 1896. He named the element after Europe, the continent where it was first found. - Occurrence
Europium is a relatively rare element, and is found in trace amounts in minerals such as bastnsite and monazite. It is also found in some uranium ores. - Applications
Europium has a number of applications, including:- Phosphors: Europium is used in phosphors for fluorescent lamps and television screens.
- Lasers: Europium is used in lasers, such as the europium-doped yttrium aluminum garnet (YAG) laser.
- Medical imaging: Europium-152 is used in medical imaging, such as bone scans.
- Importance
Europium is an important element that has a number of applications in modern technology. It is a testament to the power of scientific discovery, and it is an important part of our modern world.
Europium is one of many elements that have been named after continents. This practice is a testament to the global nature of scientific discovery, and it is a reminder that science is a collaborative effort that benefits all of humanity.
Francium - France
Francium is a chemical element with the symbol Fr and atomic number 87. It is the second-most electropositive element, after cesium, and is the heaviest alkali metal. Francium is a radioactive element with a half-life of 22 minutes, and is the heaviest known element that can be found naturally. It is named after France, where it was first discovered in 1939.
Francium is a rare element, and is found in trace amounts in uranium ores. It is also produced artificially by bombarding thorium with protons. Francium has no stable isotopes, and all of its isotopes are radioactive. The most stable isotope of francium is francium-223, which has a half-life of 22 minutes.
Francium has no practical applications, due to its short half-life and rarity. However, it is an important element for scientific research, and has been used to study the properties of radioactive elements.
The discovery of francium was an important milestone in the history of chemistry. It was the first element to be discovered that was not found naturally in significant amounts. The discovery of francium also helped to complete the periodic table, and provided new insights into the properties of radioactive elements.
Germanium - Germany
Germanium is a chemical element with the symbol Ge and atomic number 32. It is a hard, grayish-white metalloid in the carbon group, and is chemically similar to its group neighbors tin and silicon. Germanium is a relatively rare element, and is found in trace amounts in certain minerals such as germanite and argyrodite. It is also a byproduct of zinc and lead mining.
- Discovery and naming
Germanium was discovered in 1886 by the German chemist Clemens Winkler. He named the element after his home country, Germany. - Properties and applications
Germanium is a semiconductor, which means that it can conduct electricity under certain conditions. This property makes germanium useful in a variety of electronic applications, such as transistors, solar cells, and light-emitting diodes. - Importance
Germanium is an important element in the electronics industry. It is used in a wide range of devices, from computers and smartphones to solar panels and LEDs.
Germanium is just one of many elements that have been named after continents. This practice is a testament to the global nature of scientific discovery, and it is a reminder that science is a collaborative effort that benefits all of humanity.
Polonium - Poland
Polonium is a chemical element with the symbol Po and atomic number 84. It is a radioactive element with a half-life of 138 days, and is the heaviest element that can be found naturally in significant amounts. Polonium was discovered in 1898 by Marie Curie and Pierre Curie, and was named after Poland, Marie Curie's home country.
Polonium is a rare element, and is found in trace amounts in uranium ores. It is also produced artificially by bombarding bismuth with neutrons. Polonium has a variety of applications, including:
- Static eliminators
- Neutron sources
- Radioactive tracers
- Power sources for spacecraft
Polonium is a dangerous element, and can cause radiation poisoning if ingested or inhaled. However, it is also an important element for scientific research, and has been used to study the properties of radioactive elements.
The discovery of polonium was an important milestone in the history of chemistry. It was the first element to be discovered that was not found naturally in significant amounts. The discovery of polonium also helped to complete the periodic table, and provided new insights into the properties of radioactive elements.
Rutherfordium - Rutherford, New Zealand
Rutherfordium is a chemical element with the symbol Rf and atomic number 104. It is a synthetic element, and is named after Ernest Rutherford, a New Zealand-born physicist who is known as the father of nuclear physics. Rutherfordium is a radioactive element with a half-life of approximately 20 hours. It is a member of the transition metals, and is the heaviest known element in this group.
Rutherfordium is not found naturally on Earth, and is produced artificially in nuclear reactors. It is used in scientific research, and has been used to study the properties of superheavy elements.
Rutherfordium is one of several elements that have been named after continents. This practice is a testament to the global nature of scientific discovery, and it is a reminder that science is a collaborative effort that benefits all of humanity.
Dubnium - Dubna, Russia
Dubnium is a chemical element with the symbol Db and atomic number 105. It is a synthetic element, and is named after the city of Dubna, Russia, where it was first synthesized in 1967. Dubnium is a radioactive element with a half-life of approximately 16 hours. It is a member of the transition metals, and is the heaviest known element in this group.
- Discovery and naming
Dubnium was discovered in 1967 by a team of Soviet scientists led by Georgy Flerov. The element was named after the city of Dubna, Russia, where the Joint Institute for Nuclear Research is located. - Properties and applications
Dubnium is a radioactive element with a half-life of approximately 16 hours. It is a member of the transition metals, and is the heaviest known element in this group. Dubnium has no known practical applications. - Importance
Dubnium is an important element for scientific research. It has been used to study the properties of heavy elements, and has helped to expand our understanding of the periodic table.
Dubnium is one of several elements that have been named after continents. This practice is a testament to the global nature of scientific discovery, and it is a reminder that science is a collaborative effort that benefits all of humanity.
Seaborgium - Glenn T. Seaborg, USA
Seaborgium is a chemical element with the symbol Sg and atomic number 106. It is a synthetic element, and is named after Glenn T. Seaborg, an American chemist who is known for his work on the synthesis of heavy elements. Seaborgium is a radioactive element with a half-life of approximately 34 hours. It is a member of the transition metals, and is the heaviest known element in this group.
- Discovery and naming
Seaborgium was discovered in 1974 by a team of American scientists led by Albert Ghiorso. The element was named after Glenn T. Seaborg, who was the director of the Lawrence Berkeley National Laboratory at the time. - Properties and applications
Seaborgium is a radioactive element with a half-life of approximately 34 hours. It is a member of the transition metals, and is the heaviest known element in this group. Seaborgium has no known practical applications. - Importance
Seaborgium is an important element for scientific research. It has been used to study the properties of heavy elements, and has helped to expand our understanding of the periodic table.
Seaborgium is one of several elements that have been named after people. This practice is a testament to the global nature of scientific discovery, and it is a reminder that science is a collaborative effort that benefits all of humanity.
FAQs on Elements Named After Continents
The naming of elements after continents is a testament to the global nature of scientific discovery and the collaborative efforts of scientists worldwide. Here are some frequently asked questions and answers to shed light on this topic:
Question 1: What is the significance of naming elements after continents?
Answer: This practice acknowledges the contributions of scientists from various regions and celebrates the diversity of the scientific community. It also highlights the universal nature of scientific knowledge and the shared pursuit of understanding the world around us.
Question 2: Which elements are named after continents?
Answer: Americium (Americas), Europium (Europe), Francium (France), Germanium (Germany), Polonium (Poland), Rutherfordium (Rutherford, New Zealand), Dubnium (Dubna, Russia), and Seaborgium (Glenn T. Seaborg, USA).
Question 3: Who discovered these elements?
Answer: These elements were discovered by scientists from various countries, reflecting the international nature of scientific research. For example, americium was discovered by American scientists, while europium was discovered by a French chemist.
Question 4: What are the applications of these elements?
Answer: The applications of these elements vary depending on their properties. For instance, americium is used in smoke detectors, europium is employed in fluorescent lamps and lasers, and germanium finds applications in semiconductors.
Question 5: Why are some of these elements radioactive?
Answer: Elements like americium, francium, and polonium are radioactive due to their unstable atomic structures. This instability leads to the emission of radiation as the elements decay over time.
Question 6: What is the importance of studying elements named after continents?
Answer: Studying these elements provides insights into the history of scientific discovery, the properties of different elements, and their applications in various fields. It also highlights the importance of international collaboration and the contributions of scientists from diverse backgrounds.
In summary, the naming of elements after continents serves as a reminder of the global nature of scientific discovery and the collaborative efforts of scientists worldwide. These elements have diverse applications and contribute to our understanding of the periodic table and the world around us.
Transition to the next article section: Continuing our exploration of the fascinating world of elements, let's delve into the unique properties and applications of rare earth elements.
Tips on Understanding Elements Named After Continents
Understanding the elements named after continents can provide valuable insights into the history and diversity of scientific discovery. Here are a few tips to enhance your knowledge:
Tip 1: Explore the Origins of Names
Learn about the scientists who discovered these elements and the reasons behind their naming choices. This context adds depth to your understanding of the elements' history.
Tip 2: Study Their Properties
Familiarize yourself with the unique properties of each element, including its atomic number, chemical symbol, and physical characteristics. This knowledge will help you comprehend their behavior and potential applications.
Tip 3: Understand Their Applications
Discover the practical uses of these elements in various fields such as technology, medicine, and industry. Understanding their applications will highlight their importance and impact on our daily lives.
Tip 4: Appreciate the Global Nature of Science
Recognize that the naming of elements after continents reflects the international collaboration and diversity within the scientific community. This global perspective emphasizes the shared pursuit of knowledge.
Tip 5: Engage with Educational Resources
Utilize online resources, textbooks, and scientific articles to further your understanding of these elements. Engaging with multiple sources will provide a comprehensive view of the subject matter.
Summary: By following these tips, you can deepen your knowledge of elements named after continents, appreciate their significance, and unravel the fascinating stories behind their discovery and applications.
Transition to Conclusion: As we conclude our exploration, it is evident that these elements serve as testaments to the interconnectedness of science and the global contributions to our understanding of the world around us.
Conclusion
Our exploration of elements named after continents has illuminated the global nature of scientific discovery and the interconnectedness of human knowledge. These elements, each with unique properties and applications, stand as testaments to the collaborative efforts of scientists worldwide.
From americium's role in smoke detectors to europium's vibrant glow in fluorescent lamps, these elements play crucial roles in our technological advancements and daily lives. Their names, derived from the continents where they were discovered or the scientists who isolated them, serve as reminders of the diverse origins of scientific knowledge.
As we continue to unravel the mysteries of the natural world, it is essential to embrace the spirit of global collaboration and recognize the contributions of scientists from all corners of the globe. The naming of elements after continents is not merely a geographical designation but a celebration of the collective human endeavor to understand and harness the power of nature.
Uncover The Personal And Professional Journey Of Cindy Busby: Motherhood And Its Impact
Discover The Unbreakable Bond: Unveiling The Secrets Of Juju Watkins' Partner
Unveiling The Height Of Destin Conrad: Insights And Surprises Revealed

ncG1vNJzZmifkZi8s76NmqSsa16Ztqi105qjqJuVlru0vMCcnKxmk6S6cLHLnqSepqRiu6K5xJ1kmp6kmr9urYycpqesmaOyr8CNoaumpA%3D%3D